Wetlaid Leggings
From €0.00

Medline’s Wetlaid Leggings give the end user flexibility of use during a variety of surgical procedures. Another advantage is the high level of comfort and breathability afforded, thanks to the use of a non-woven material made from cellulose.
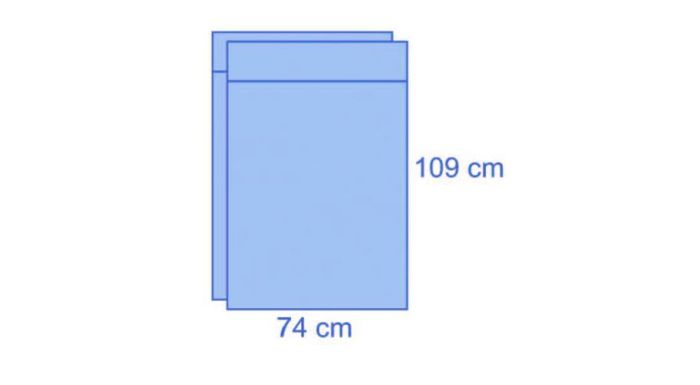
The two leggings have dimensions of 74 x 109 cm and integrate a 15-cm cuff.
Medline’s surgical drapes and packs are developed, tested and trusted by operating theatre professionals. Our surgical drapes offer the best available features and are fully compliant with the EN13795 standard. Our intuitive labelling system displays informative product images and detailed specifications.
The two leggings have dimensions of 74 x 109 cm and integrate a 15-cm cuff.
Medline’s surgical drapes and packs are developed, tested and trusted by operating theatre professionals. Our surgical drapes offer the best available features and are fully compliant with the EN13795 standard. Our intuitive labelling system displays informative product images and detailed specifications.
Contact Us
How to order
Select Items
SKU:
DYNJPE2460
Pack Version
Initial
Unit Price:
€0.00
Pack Size:
20
Select quantity:
Case
Line Price:
0.00€
Total
0.00€
Contact Us
How to order
Step 1 - Select a SKU
Step 2 - Select a List
Back
Description
Medline’s Wetlaid Leggings give the end user flexibility of use during a variety of surgical procedures. Another advantage is the high level of comfort and breathability afforded, thanks to the use of a non-woven material made from cellulose.
The two leggings have dimensions of 74 x 109 cm and integrate a 15-cm cuff.
Medline’s surgical drapes and packs are developed, tested and trusted by operating theatre professionals. Our surgical drapes offer the best available features and are fully compliant with the EN13795 standard. Our intuitive labelling system displays informative product images and detailed specifications.
The two leggings have dimensions of 74 x 109 cm and integrate a 15-cm cuff.
Medline’s surgical drapes and packs are developed, tested and trusted by operating theatre professionals. Our surgical drapes offer the best available features and are fully compliant with the EN13795 standard. Our intuitive labelling system displays informative product images and detailed specifications.
Specification
| EN13795 Level | High Performance |
|---|---|
| Main Material Feature | Repellent and Breathable |
| Adhesive | No |
| Packaging | High Performance |
| Type of Product | Drapping Pack |
| Main Material | Cellulose |
| Fluid Collection Pouch | No |
| Colour of surgical drape | Blue |
| Single Use | Yes |
| Sterile | Yes |
Downloads
Ordering Information
| SKU ◣ | Pack Version | Qty per case |
|---|---|---|
| DYNJPE2460 | Initial | 20 |